New Drugs and New Therapies Currently in use or in
Clinical Trials that may be used for Ewing's Sarcoma and other Pediatric Cancer
Metastasis or Relapse:
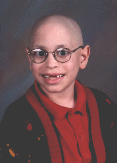


This site maintained by Barry Sugarman, B.S.ENGR.
Father of Alon Sugarman, Diagnosed March 6, 1998
with Ewing's Sarcoma of the Distal Femur.
E-Mail to:
barry@cureourchildren.org
** Warning: These drugs should be discussed with
your primary physician and an oncologist prior to use. These drugs are
only available upon prescription of a licensed medical doctor. These
drugs are for those patients who have already completed the standard drug
regimen for Ewing's Sarcoma and have had a reoccurrence of the disease, or those
who are participating in a clinical study, or those who are otherwise requested
to do so by their treating physician or oncologist. The standard drug
regimen products are listed at the bottom of our home
page. The standard drug regimen has been tested in
large, long term clinical trials, and is the most effective proven regimen known
at this time. The information herein does not constitute a medical
opinion. **
Treatment Rating System:
Category 1: Front Line, First Choice Treatments for
Ewing's Relapse. Substantial Scientific Evidence of Safety and
Efficacy. All drugs are FDA approved. These regimens known to be in
used as a first choice treatment plan in relapse by reputable oncologists.
Category 2: Substantial Scientific Evidence of Safety of the individual
drugs in the regimen or of the individual or combination of drugs in
adults. These regimens are also known to be in use by reputable
oncologists when other first choice therapies have failed. These are
primarily New Regimens and Combinations of Traditional Chemotherapy with New
Agents for Treatment of Ewing's Relapse.
Category 3: Some evidence of safety and efficacy, but still in clinical
trials, and known to be in use by reputable oncologists. Not currently approved
by the FDA yet.
Category 4: No proven evidence of safety or efficacy in humans currently,
in Phase I clinical trials.
Category 5: Developmental and highly speculative or theoretical.
General Reference Links:
American
Society of Clinical Oncologists (ASCO) Pediatric Solid Tumor Abstracts 2002,
Phone: 703-299-0150, Fax: 703-299-1044
American
Society of Clinical Oncologists (ASCO) Pediatric Solid Tumor Abstracts 2001,
Phone: 703-299-0150, Fax 703-299-1044
American
Society of Clinical Oncologists (ASCO) Abstracts 2001, Pediatric
Leukemia/Lymphoma, Phone: 703-299-0150, Fax: 703-299-1044
American
Society of Clinical Oncologists (ASCO) /Search Abstracts for all 1995 to
present/, Phone: 703-299-0150, Fax: 703-299-1044
European Society
of Medical Oncology Abstracts, Lugano, Switzerland, Phone: 011-41-91-973-1900,
Fax 011-41-91-973-1902
Biospace Drugs
in Clinical Development Search Engine, Phone: 888-BIOSPACE or 415-355-6500
Category 1: Front Line, First Choice Treatments for Ewing's
Relapse. Substantial Scientific Evidence of Safety and Efficacy.
All drugs are FDA approved. These regimens known to be in used as a first
choice treatment plan in relapse by reputable oncologists:
2/24/2001--Ifosfamide, Etoposide, Carboplatin (ICE) or Ifosfamide,
Etoposide, or Ifosfamide or Docetaxel or Irinotecan with
Vincristine or Topotecan with Cyclophosphamide. These are the
front line, first choice treatments for Ewing's relapse chosen by oncologists
most experienced with Ewing's Sarcoma. Once the tumor is under control again,
high dose chemotherapy with stem cell rescue may be helpful and should be
discussed with the oncologist. Please also see our foundation Stem
Cell Transplant Page for more information. Doctors utilizing these
regimens include Dr. James Miser, City of Hope National Medical Center, Phone
800-535-7119, email: jmiser@coh.org,
Professor Stuart Siegel, Children's Hospital of Los Angeles, Phone is
323-669-2205, email:ssiegel@chla.usc.edu
and Dr. Doug Hawkins at the University of Washington, Seattle, Phone:
206-526-2106 or (206) 526-2131, email:dhawki@chmc.org
Also, Paul Meyers, M.D.,
Memorial Sloan Kettering Cancer Center, Phone: 212-639-5952, Fax:
212-717-3447 Email:meyersp@mskcc.org
ICE Supporting Research: Here are published research abstracts
from: ASCO,
and Medline.
Topotecan with Cyclophosphamide Supporting Research: Abstracts: ASCO
and Medline.
Hycamtin®
(generic name Topotecan), Manufactured by Glaxo
SmithKline, Phone: 1-888-825-5249 or 1-412-928-1000 with Cytoxan®
(generic name Cyclophosphamide) Manufactured by Bristol
Meyers Squibb, Phone: 800-321-1335 or 800-468-7746 or 212-546-4000. A Phase
II study was conducted by the Pediatric
Oncology Group, which merged into the Children's
Oncology Group. The protocol is
here. Title: Cyclophosphamide Plus Topotecan In Children with
Recurrent or Refractory Solid Tumors (POG 9464) (HSC 970117)(HSC 990221).
Principal Investigator: Sharon B. Murphy, Chair, Ph: 773-880-4562, Pediatric
Oncology Group. This trial was able to enroll 91 patients, and the results were
favorable; the
published results abstract are here.
Taxotere® (generic name Docetaxel)
manufactured by Aventis
Pharmaceuticals, Phone: 800-633-1610 or 908-243-6000. Taxotere
web site is here. Status: FDA approved in the United States for
locally advanced or metastatic breast cancer. In use as a single agent for
relapsed Ewing's Sarcoma by Dr. Doug Hawkins at the University of Washington,
Seattle, Phone: 206-526-2106 or (206) 526-2131, Fax: 206-527-5182, email:dhawki@chmc.org
and by Dr. Andrew Pendleton at Cabell Huntington Hospital in Huntington, West
Virginia, Phone 304-691-1300. The abstract of one Phase I trial of
primarily osteosarcoma patients is
here. The complete published journal article is
here. The abstract of another Phase I trial conducted at the National
Institute of Health is
here.
Camptosar®
(generic name Irinotecan [CPT-11]) manufactured by Pharmacia
Upjohn Co. Phone: 800-800-323-4204. There is a recently completed Phase I
clinical study in children with relapsed solid tumors with good results. The
study was conducted at St.
Jude Children's Research Hospital in Memphis,
Tennessee, and The University of Tennessee also in Memphis. The Study
synopsis appears in the Proceedings of the American Society of Clinical
Oncology, Volume 17, 1998, No. 721. The contact at St. Jude is Wayne L.
Furman, M.D. Phone 901-495-2403/3577, mailto:wayne.furman@stjude.org
**Professor Stuart Siegel, M.D. at Children's
Hospital of Los Angeles also reports good preliminary results on
patient with relapsed Ewing's Sarcoma. His Phone is 323-669-2205, mailto:ssiegel@chla.usc.edu
Status: FDA approved drug but not for Ewing's Sarcoma.
*Added 12/28/2001* Cosmegen®
(generic Name Dactinomycin) -- Manufactured by Merck
& Co., Phone: 1-866-448-7590. This drug is used in Europe instead
of Adriamycin (doxorubicin) in the initial treatment protocol. This drug
may be used as a substitute for doxorubicin for those patients who have reached
their doxorubicin limit, or may be used alone or in combination with other
chemotherapeutic agents for Ewing's relapse.
Thalmomid® (Thalidomide)--Manufactured by Celgene
Corporation, Phone: 732-271-1001, FAX 732-271-4184, mailto:webmaster@celgene.com,
Status: FDA approved drug, but not FDA approved for Ewing's Sarcoma. May
be prescribed for any use by a licensed medical doctor.
Category 2 Chemotherapeutic Agents : Substantial Scientific Evidence
of Safety of the individual drugs in the regimen or of the individual or
combination of drugs in adults. These regimens are also known to be in use
by reputable oncologists when other first choice therapies have failed.
These are primarily New Regimens and Combinations of Traditional Chemotherapy
with New Agents for Treatment of Ewing's Relapse:
*New* 7/14/2001--Mitroxantrone
(Novantrone®) and Interferon
Beta 1B (Betaseron®). This is reported to be in use for Ewing's
relapse at M.D. Anderson
Cancer Center, Phone: 800-392-1611, 713-792-6161 via Dr.
Carl Plager, Phone: 713-792-3626, Fax: 713-794-1934. Both drugs
are FDA approved treatments for other conditions. Mitroxantrone is
approved for hormone refractory prostate cancer, acute myleogenous leukemia
(AML), and Multiple Sclerosis (MS). Manufacturer of Novantrone is Angen,
Inc., Phone: 805-447-1000 or 805-447-1010. Manufacturer of
Interferon Beta 1B is Berlex
Laboratories, Inc., Phone: 888-BERLEX4 or 973-276-2000.
*New* 6/26/2001--Topotecan
(Hycamtin®) and Irinotecan
(Camptosar®)[Also called: CPT-11]. Phase I Study of
the combination in Children with chemotherapy resistant tumors has been
conducted and reported by the oncologists at St.
Jude Children's Research Hospital, Phone: 901-495-3300 under
this protocol. Manufactures of the drugs are: Glaxo Smith Kline (Hycamtin),
Phone: 1-888-825-5249 or Pharmacia
and Upjohn (Camptosar), Phone: 1-800-253-8600 x8244.
*Updated 7/1/2001* Gleevec
(Imatinib mesylate) is a novel FDA approved compound approved
for use in patients with chronic myeloid leukemia (CML).
The results in CML have been excellent in over 90% of the cases and are
dramatic. The drug apparently inhibits several enzymes that are present in
some types of tumors including the Bcr-abl enzyme, the CD117 enzyme (C-kit), and
PDGRF, Platelet derived growth factor. Reputable oncologists such as George
Demetri, M.D. at Dana-Farber
Cancer Institute, Phone: 617-632-5122, Email:SarcomaDoc@aol.com,
and Warren
Chow, M.D. at City of
Hope National Medical Center, Phone: 800-535-7119 are known to be testing
their sarcoma patients for the presence of CD117 (c-kit) enzyme, and if the test
is positive, and other chemotherapy has not worked, they are administering this
drug. A
further explanation of this is available here. The manufacturer of the
drug is Novartis,
Phone: 888-NOW-NOVA.
*New* 6/24/2001--Doxorubicin
HCl Liposome (Doxil®). Phase I Study of Doxorubicin HCl
Liposome in Pediatric Patients With Refractory Solid Tumors, Protocol
Numbers: NCI-99-C-0039D, LIPO-NCI-99-C-0039, NCI-99-C-0039, Frank Milton
Balis, Principal Investigator, Phone: 301-496-0085, Pediatric
Oncology Branch, National Institute of Health, Bethesda, Maryland, Frank Milton
Balis, Principal Investigator, Ph: 301-496-0085, Children's
Hospital of Philadelphia Philadelphia, Pennsylvania 19104, Phone: 1-800-879-2467
or 215-590-1000, Manufacturer
is Alza Corporation., Phone: 650-564-5000, Fax: 650-564-7070. Product
Web Site is http://www.doxil.com
*New* 6/24/2001--Trastuzumab
(Herceptin®).
Currently FDA approved for metastatic breast cancer where the tumor over
expresses the HER-2 or HER-2/neu Protein. Has the possibility of severe
side effects including cardiac toxicity and allergic reaction, see the product
insert. Investigational only for other tumors, but should only
be used when the tumor is tested for HER-2 or HER-2/neu Protein over
expression. Manufacturer is:
Genentech, Inc., Phone: 650-225-1000, Fax: 650-225-6000.
2/24/2001--Low dose Cyclophosphamide or Vinblastine in combination
with Celecoxib (Celebrex®). Sylvain Baruchel, M.D.,
Associate Professor & Senior Associate Scientist, Director, New Agents and
Innovative Therapy in Oncology, Phone: (416) 813-7795, Fax: (416) 813-5327, E-mail:
sylvain.baruchel@sickkids.on.ca at the Hospital
for Sick Children in Toronto, Canada.
*Updated 6/29/2001* Thalidomide
with Cyclophosphamide, Phase II Study, Protocol
numbers NCI-G99-1498, MSKCC-98088, MSKCC-98088A(1). Ira Dunkel, Principal
Investigator, Phone: 212-639-2153, Memorial
Sloan-Kettering Cancer Center, New York, New York, U.S.A., Phone: 800-525-2225,
or 212-639-4900. The ASCO abstract of this study
is here.
Taxotere® (generic name Docetaxel) with
Carboplatin, Currently in study in Germany under this
protocol. And is under study in Greece under this
protocol. Taxotere is manufactured by Rhone-Poulenc Rorer. Taxotere
web site is here. The results of a German study are
here. Free Medscape registration is required to view the article, or you can
email the author: Dr. W. Schuette, Department of Internal Medicine II, City
Hospital Martha-Maria, Röntgenstrasse 1, D-06120 Halle, Germany. E-mail: wolfgang.schuette@medizin.uni-halle.de
Phone: 011-49-0345-557-4480, Fax: 011-49-0345-557-4484.
*Updated* 11/23/2002--Oxaliplatine
(Eloxatin®).
Recently approved in the USA and approved in Europe. Ongoing clinical trials
here in the United States for numerous malignancies including solid
tumors. This compound is in the same class as carboplatin and other
platinum containing compounds. Phase
I Study of Oxaliplatin in Children With Advanced Solid Tumors, Protocol Numbers:
SJCRH-OXAL1, NCI-T99-0059, Charles Benton Pratt, III, Principal
Investigator, Phone: 901-495-3442, Saint Jude Children's Research Hospital,
Memphis, Tennessee, U.S.A., Numerous other studies ongoing listed at http://www.cancer.gov/search/clinical_trials,
Phone: 800-422-6237, Manufacturer
is Sanofi-Synthelabo, France, Phone: 011-33-0-1-53-77-42-23, Fax:
011-33-0-1-53-77-42 65 . Please note that platinum containing products are
toxic to the ears, and may cause permanent hearing loss or deafness.
Category 3 Chemotherapeutic Agents: Some evidence of safety and
efficacy, but still in clinical trials, and known to be in use by reputable
oncologists. Not currently approved by the FDA yet:
*New 12/28/2001* Phase
II Study of Nonmyeloblative Allogeneic Peripheral Blood Stem Cell
Transplantation and Donor Lymphocyte Infusions in Patients With
Refractory Metastatic Solid Tumors. An interesting new study for those who
cannot tolerate high dose myleoblative chemotherapy. This procedure
involves using donor stem cells to try to get the body to generate an immune
response against the tumor. Available only at the National Institute of
Health under Protocol
ID No. NHLBI-99-H-0064. Richard W. Childs, Chair Ph: 800-411-1222,
National Heart, Lung, and Blood Institute.
*Updated 6/29/2001* DX-8951f
is a novel and potent topoisomerase I inhibitor, water-soluble camptothecin
derivative, currently in phase I clinical trials. A clinical trial was
recently conducted with significant results at St.
Jude Children's Research Hospital, Phone: 901-495-3300 under
this protocol. A
preliminary in-vitro study indicates that the drug is effective in
adult and pediatric cancers. Phase I and phase II studies are ongoing at
the San
Antonio Cancer Institute. Contact is Stephanie Hodges, R.N., Patient
Referral Coordinator, Phase I Department, Grossman Cancer Center, 7979
Wurzbach Road, San Antonio, TX 78229, Phone 210-616-5798, FAX 210-616-5799, mailto:shodges@saci.org.
Manufacturer is Daiichi
Pharmaceutical Corporation, Phone: 877-324-4244 or 201-573-7000.
*Updated 7/1/2001* Epothilone
A and B are novel and potent compounds derived from the bacteria
Sorangium. Epothilone B is currently under development by Novartis,
Phone: 888-NOW-NOVA under the name EPO906. An article about them is
here. An abstract of the Phase I study is
here and another is
here. One study was conducted at Princess
Margaret Hospital in Toronto, Canada, Phone: 416-340-3388, and The
Cancer Institute of New Jersey, New Brunswick, New Jersey, Phone: 732-235-CINJ.
Another study was conducted in England at Beatson
Oncology Centre, Glasgow, UK, Phone: 011-44-141-330-3953, Fax:
011-44-141-330-4127; Northern
Centre for Cancer Treatment, Phone: 011-44-191 273 8811, Newcastle upon Tyne, UK.
Another epothilone analog is called BMS-247550 under development
by Bristol
Meyers Squibb, Phone: 212-546-4000. An abstract on this compound is here.
It is under study at Albert
Einstein College of Medicine, Bronx, NY, Sridhar Mani, Principal
Investigator, Phone: 718-904-2488 under
this protocol; and NYU
Medical Center, New York, NY, Phone: 212-263-7300. Currently in Phase
II for the treatment of non-small cell lung cancer in people who have failed
first-line platinum-based chemotherapy.
*New* 7/14/2001--Lung
Cancer Vaccine (GVAX®).
These vaccines are made of tumor cells taken from individual patients that
have undergone irradiation and genetic modification so that they secrete a
hormone that helps stimulate the immune system. Research is ongoing
at Stanford
University Medical Center, with the lead doctor of Dr. Lawrence Fong,
Phone: 650-723-7621, Fax: 650-723-1399. Manufactured by Cell
Genesys Inc., Phone: 650-425-4400, Fax: 650-425-4457. The
current study is entitled: Phase I/II Study of GM-CSF Gene-modified Autologous
Tumor Vaccines in Early and Advanced Stage Non-Small Cell Lung Cancer (NSCLC),
and is ongoing nationwide.
ImmTher® Manufactured by Endorex
Corp., Phone: 847-573-8990, FAX: 847-573-9285, mailto:mrosen@endorex.com
Status: In Phase II clinical trials for Ewing's Sarcoma. FDA orphan drug
status designation for Ewing's Sarcoma and Osteosarcoma.
Unique Treatments:
Category 2: Substantial Scientific Evidence of Safety of the
individual treatments in the regimen or of the individual or combination of
drugs in adults. These regimens are also known to be in use by reputable
oncologists when other first choice therapies have failed. These are
primarily New Regimens and Combinations of Traditional Chemotherapy with New
Agents for Treatment of Ewing's Relapse.
*New* 7/3/2001--Radio
Frequency Ablation, This novel treatment
is currently approved by FDA for treating non-resectable liver lesions; however,
it can be used for soft tissue such as breast, lung and kidneys. The
manufacturer of the device is Boston
Scientific Corporation, Toll-free: 800-225-3226, Phone: 508-650-8000, Fax:
508-650-8923. Another write-up on this procedure is
here. This procedure basically destroys tumor cells locally by
submitting them to radio frequency waves.
*New* 6/24/2001--Samarium
Sm 153 Lexidronam (Quadramet®), Although this
compound is approved by FDA for cancer bone pain, it is being used successfully
to treat tumors. Samarium-153 emits both medium-energy beta
particles and a gamma photon, and has a physical half-life of 46.3 hours (1.93
days). Quadramet (samarium Sm-153 EDTMP) has an affinity for bone and
concentrates in areas of bone turnover in association with hydroxyapatite. In
clinical studies employing planar imaging techniques, more Quadramet accumulates
in osteoblastic lesions than in normal bone with a lesion-to-normal bone ratio
of approximately 5. The mechanism of action of Quadramet in relieving the pain
of bone metastases is not known. Under study in local protocols by: Steven
P. Howard, MD, PhD, Principal Investigator, Phone: 608-263-8500, University
of Wisconsin Comprehensive Cancer Center, Manufacturer
of this compound is: Berlex Laboratories, Inc., Phone: 1-888-BERLEX4 or
973-276-2000.
*New* 11/25/2002--Bipolar
Radiofrequency Interstitial Thermo Therapy (RFITT). This is another
unique procedure that utilizes radiofrequency and minimally invasive techniques
for tumor treatments. A company developing this technology is Celon
AG in Germany, Celon AG medical instruments
Rheinstr. 8, D-14513 Teltow b. Berlin, Germany, Phone: 011-49-3328-3519-0,
e-mail info@celon.com
Celon USA, Inc., P.O. Box 445 Spring Park, MN 55384-0445 USA, Fax:
952-495-0067
*New* 11/28/2000--High
Intensity Focused Ultrasound (HIFU). This is a unique procedure
recently developed and primarily used for prostate cancer, but now being tried
on other localized cancers. The tumor is heated up with ultrasound until
the tissue is killed. One machine is made by a company named Misonix,
Phone: 800-645-9846. One of the clinical trials is being conducted at
Indiana at Focus Surgery
Inc.(FSI), Phone: (317)541-1580. This is a private company based in
Indianapolis, Indiana founded in 1996 and has collaborated in research and
development of High Intensity Focused Ultrasound (HIFU) with the Indianapolis
Center for Advanced Research (ICFAR), and the Indiana University School of
Medicine. As with any investigational device, compassionate use for other
non-prostate indications may be available. Another company, EDAP
Technomed, Phone: (770) 446-9950 is also developing a device currently in
use for prostate cancer and being
developed for breast cancer and other malignant tumors. There is
additional evidence regarding HIFU
in liver tumors here and also
in this abstract. An article from ABC News about this
procedure is
here. Another article on HIFU is
here.
8/6/2000--Photodynamic Therapy (PDT). This is a U.S. Food and
Drug Administration approved procedure. This procedure involves
intravenous injection of a drug that accumulates in cancerous cells, and
insertion of a laser device in the mouth to the lung area where laser light is
directed to the tumor sites which activates the drugs, and kills the tumor
tissue. The current approved drug is Photofrin
(Porfimer Sodium) marketed by Axcan
Scandipharm Inc., 22 Inverness Center Parkway, Birmingham, Alabama 35242,
(205) 991-8085 / (800) 950-8085 / Fax (205) 991-9547 in the United States. A
current approved manufacturer of the laser is Laserscope,
3052 Orchard Drive, San Jose, CA 95134-2011, Phone 408-943-0636.
Another approved manufacturer is Diomed
Lasers in England. Phone: +44 1223 729340, Fax: +44 1223 729329.
Category 3: Some evidence of safety and efficacy, but still in
clinical trials, and known to be in use by reputable oncologists. Not currently
approved by the FDA yet.
*New 12/29/2001* AptosynTM
(Exisulind). Aptosyn™ is the first of a new class of compounds
called Selective Apoptotic Antineoplastic Drugs (SAANDs). Aptosyn™ selectively
induces apoptosis (programmed cell death) in neoplastic cells by inhibiting the
enzyme cyclic GMP-phosphodiesterase (cGMP-PDE). developed by Cell
Pathway, Inc., 702 Electronic Drive, Horsham, PA 19044, Phone: 215-706-3800,
Fax: 215-706-3801, Email:trials@cellpathways.com.
A Phase I study in combination with Camptosar®
(generic name Irinotecan [CPT-11]) manufactured by Pharmacia
Upjohn Co. Phone: 800-253-8600 x8244. was conducted by Dr. John L. Marshall
of the Vincent T.
Lombardi Cancer Center of Georgetown University Medical Center in Washington,
DC., Phone 202-784-4000.
*New* 6/24/2001--Holmium-166
DOTMP, This novel radiopharmaceutical is
holmium-166-DOTMP. It emits high-energy beta particles that can destroy cells.
DOTMP binds to bone and carries holmium to the bone and adjacent bone marrow.
Because radiation is selectively delivered to the site of the disease, the
exposure to normal organs is reduced compared to conventional external beam body
irradiation. This agent is designed to treat cancers that arise in the bone or
bone marrow, as well as those that spread to the bone and bone marrow, such as
breast and prostate cancers. Phase I/II Study, Protocol
numbers FHCRC-1474.00, NCI-G00-1842, CHMC-S-6007, Doug Hawkins, M.D.,
Principal Investigator, Phone: 206-526-2100, Fax 206-527-3946, email:dhawki@chmc.org,
Children's Hospital and Regional
Medical Center, Seattle, Manufacturer
of this compound is: NeoRx Corporation, Phone: 206-281-7001, Fax:
206-284-7112.
*New* 11/28/2000--Dendritic
Cell Vaccine (Immune System Enhancement). This is a new unique
procedure currently under study at the University of Michigan, CS
Mott Children's Hospital, Phone: 1-800-211-8181. The doctors
conducting the study are Dr. Jim Geiger and Dr. Raymond Hutchinson under
protocol number UMCC9702. Their research nurse is Cynthia Bower, Phone:
734-615-5294. Another doctor at the University of Michigan, Dr. Laurence
H. Baker is also studying Dendritic Cell Vaccines. His phone number is
734-936-8456. Their Cancer Information Line is 800-865-1125. This is
the link to the General
Clinical Research Center. That protocol is
here. There are recent articles on this therapy at this
location and also here.
This type of vaccine is also available from the University
of Pennsylvania, Phone: 1-800-789-PENN. Duke
University, Phone: Renee Twombly 919-684-4148 also has studies ongoing. So
does Dana Farber in coordination with Genzyme
Molecular Oncology Phone: 508-872-8400, Fax: 508-271-2604 according to one
press release. An NIH abstract on this technology is here.
Investigational Drug Listing:
Category 4: No proven evidence of safety or efficacy in humans
currently, in Phase I clinical trials.
*New 12/29/2001* ThymitaqTM
(Nolatrexed Dihydrochloride). This is a novel inhibitor of thymidylate
synthase developed by Eximas
Pharmaceutical Corporation, 1055 Westlakes Drive, Suite 200, Berwyn, PA 19312,
Phone: 610-560-0600, Fax: 610-560-0700. A Phase I study was conducted
in children with advanced cancer by E.J. Estlin of the United Kingdom Children's
Cancer Study Group, Cancer Research Unit, University
of Newcastle upon Tyne, United Kingdom, Phone: 011-44-191 202 3033, Fax:
011-44-191 202 3060. One patient with spinal PNET had a favorable
response.
*New 12/28/2001* Halofuginone
(Angiogenesis Inhibitor). This is a novel anti-angiogenesis drug that
is derived from a plant and developed in Israel by Collgard
Biopharmaceuticals Ltd., 233 Needham Street, Newton, MA 02464, Phone:
617-454-1016, Fax: 617-454-1026. In Israel: 1B Bazel Street, Kiriat Arie,
Petah Tikva 49170 Israel, Phone: 011-972-3-924-7477, Fax 011-972-3-972-7446,
Neal Farber, President/CEO, Email:farber@collgard.com
*New* 11/28/2000--PS-341
(Proteasome Inhibitor). This is a novel new drug that affects the cell
protein degradation pathway. I have information that some doctors at Massachusetts
General Hospital, Phone: 617-726-2070 and the University
of North Carolina at Chapel Hill, Phone: 919-966-6047 are now trying this
drug in Ewings patients. This drug is under formal study in Phase I
clinical trials in the United States at the Dana
Farber Cancer Institute, Phone:800-733-4662, the University
of Texas M.D. Anderson Cancer Center, Phone: 1-800-392-1611, Memorial
Sloan-Kettering Cancer Center, Phone: 1-800-525-2225 and Mayo
Clinic Cancer Center, Phone: 1-800-525-2225. Preliminary
Phase I results are here. An
article on the drug is here. There is more information on this drug here.
Manufacturer is Proscript
Inc, Phone: 617-374-1470 which is now owned by LeukoSite,
Inc/Millennium Pharmaceutical (NasdaqNM:MLNM).
8/6/2000--Zadaxin (Thymosin alpha 1). This drug is under
study in the United States, but it is approved in 20 countries for treatment
hepatitis B and C, and now a study exists for treatment of cancer. The
study abstract is here. There is more information on this drug here.
Manufacturer is Sciclone
Pharmaceuticals (United States). The International Site is at Sciclone
International.
7/8/2000--Tumor Necrosis Factor alpha (TNF-alpha). This
drug is under study in the United States at the NIH under protocol
no. NCI-99-C-0145. There are new studies
in soft tissue sarcoma showing effectiveness here. Manufacturer
is Vion Pharmaceuticals Inc.
6/6/2000--Aplidin. There is a study abstract at this
location. Here
is a brief profile of this drug in Phase II clinical trials. There are
several Medline Abstracts: 1,
2,
and 3.
May be available under compassionate use guidelines. Manufacturer
is PharmaMar.
5/20/2000--Combretastatin A4 Prodrug (CA4P).
Antiangiogenesis inhibitor class compound. This drug is manufactured by
Oxigene, Inc., One Copley Place, Boston, MA 02116, Phone 617-673-7800, FAX
617-924-9229, See also: http://www.oxigene.com
May be available under compassionate use. ***Oxigene is positioning the product
to be used against a wide variety of solid tumors. Click
here for the more information on Combretastatin.
5/20/2000--Decitabine. This drug is manufactured by
Supergen, Inc., Two Annabel Lane, Suite 220, San Ramon, CA 94583, Phone
925-327-0200, FAX 925-327-7347, mailto:investor_relations@supergen.com,
See also:http://www.supergen.com
May be available under compassionate use. ***Supergen is positioning the product
to be used against a wide variety of solid tumors, and has entered into an
agreement with the National Institute of Health to start Clinical trials. Click
here for the press release.
5/20/2000--Apra (CT-2584). This drug is manufactured by
Cell Therapeutics, Inc. 201 Elliot Avenue West, Suite 400, Seattle, WA
98119-4230, Phone 206-282-7100, FAX 206-284-6206, invest@cticseattle.com,
See also:http://www.cticseattle.com
Undergoing clinical trials for sarcomas, also may be available under
compassionate use.
5/20/2000--RFS-2000 (Rubitecan). This drug is
manufactured by Supergen, Inc., Two Annabel Lane, Suite 220, San Ramon, CA
94583, Phone 925-327-0200, FAX 925-327-7347, mailto:investor_relations@supergen.com,
See also:http://www.supergen.com
May be available under compassionate use. Supergen is positioning the product to
be used against a wide variety of solid tumors.
4/16/2000--Betulinic Acid. This is a very new cytotoxic
agent drug with promising results against Ewing's Sarcoma, Neuroblastoma,
Medulloblastoma, and Glioblastoma. It is being developed in Germany. The
contact information is: Klaus-Michael Debatin, University Children's Hospital,
Prittwitzstrasse 43, D-89075 Ulm, Germany, Phone 011-49-731-502-7700, FAX
011-49-731-502-6681, mailto:klaus-michael.debatin@medizin.uni-ulm.de.
Dolastatin 10 A linear polypeptide that is a unique
and extremely potent antimitotic agent. This drug is isolated the Sea
Hare, a marine mollusk. A brief article is here.
Status: Currently in Phase II clinical trials all over the country under this master
protocol with Principal Investigator: Hedy L. Kindler, MD, Protocol
chair, University of Chicago Cancer Research Center, Phone: 773-702-0360 or
1-888-824-0200.
*Updated 3/6/2001* Ecteinascidin
743 (ET-743), YondelisTM isolated from a tunicate,
Ecteinascidia turbinata, collected in the Caribbean. An anticancer drug
that inhibits the growth of cancer cells by disrupting the structure of tumor
cell DNA. Status: Currently in Phase I trials here in the USA, Canada and
Austrailia under this
protocol. The chair of this protocol is: Sylvain Baruchel, Chair,
Children's Oncology Group, Phone: 416-813-7795. Also, currently in Phase
II clinical trials in Europe under these protocols for Sarcoma.
The chair is Ole Steen Nielsen, Chair, Phone: 1945-89-49-2555, EORTC Soft Tissue
and Bone Sarcoma Group. Another study is in Phase II for Osteosarcoma.
The chair is Paul A. Meyers, Principal Investigator, Phone: 212-639-5952,
Memorial Sloan-Kettering Cancer Center, New York, New York, U.S.A. The
manufacturer is PharmaMar
S.A., in Spain, Phone: 34 (91) 803.2000, Fax: 34 (91) 803.1143. In the
United States: PharmaMar USA, 320 Putman Ave., Cambridge, Massachussets 02139
USA, Phone: 617-868-3797, Fax: 617-868-0109.
Vaccine Therapy An immune system therapy being conducted
at the National
Institute of Health. See the following protocols at:
NIH
Vaccine Therapy, Protocol No. NCI-97-C-0052K, NCI-T96-0038
NIH
Vaccine Therapy Protocol No. NCI-97-C-0050J, NCI-T96-0037
NIH
Vaccine Therapy Protocol No. MSKCC-99077, NCI-H00-0052
Crystal Mackall, Chair, Phone: 301-402-5940, Division of Clinical Sciences
Jonathan Lewis, Chair, Phone: 212-639-2940, Memorial Sloan-Kettering Cancer
Center Status: In Clinical Trials
Angiostatin® , Endostatin®,
and 2-Methoxyestradiol Manufactured by EntreMed
Inc. Phone: 301-738-2490, FAX 301-738-2490 Status: In Clinical
Trials. Contact for Endostatin Trials is: Phone 617-632-5100. Contact for
Angiostatin Trials is Phone 1-800-JEFF-NOW
Endostatin Trials at Dana
Farber Cancer Institute at Harvard
Angiostatin
Trials at Thomas Jefferson University Hospital
NCI
Table of Clinical Trials for Antiangiogenesis Drugs
SU6668®, SU11248®, and SU101®
and (Angiogenesis Inhibitors)--Manufactured by Sugen,
Inc, a Pharmacia Company., Phone: 866-804-1837 or 650-553-8300, FAX:
650-553-8301, mailto:ir@sugen.com Status:
In clinical trials.
Proleukin® (Aldesleukin, IL-2)--Manufactured
by Chiron Corporation,
Phone: 510-655-8730, Fax: 510-655-9910. mailto:corpcomm@cc.chiron.com,
Status: FDA approved drug, but not approved for Ewing's Sarcoma. May be
prescribed for any use by a licensed medical doctor.
Category 5: Developmental and highly speculative or theoretical.
7/7/2000--Lovastatin (Mevacor®). This drug is
approved already in the United States and is widely used to reduce
cholesterol. There are new, preliminary lab studies that show activity in
Ewing's Sarcoma and other cell lines. There is a study abstract at this
location. Manufacturer is Merck
& Co.
4/16/2000--White Blood Cell Transfusion from matching donor.
This technique is being sponsored by Kenneth M. Tokita, M.D., Cancer Center of
Irvine, 16100 Sand Canyon Avenue, Irvine, California 92618, Phone 949-417-1100,
FAX 949-417-1165, mailto:med@ccoi.org. The
company web site is at: http://www.ccoi.org
Capoten® (generic name Captopril Manufactured by Bristol
Meyers-Squibb)
and Abbokinase® (generic name urokinase Manufactured by Abbott
Laboratories) combination. This is an antiangiogenesis combination developed
by Dr. Hans-Christoph Rossbach and described in a Chicago
Tribune article on 5/8/99. To reach Dr. Rossbach: Phone: 800-456-4543
or 727-892-4176, FAX 727-892-4379 Status: Both Drugs are FDA approved but
not for Ewing's Sarcoma. A preliminary trial of one patient with reoccurring
Ewing's has been treated successfully.
*New* 4/16/2000--Another physician who has started a study
using this protocol is Dr. Gearld A. Soff, M.D., Northwestern Memorial Hospital,
Galter 4-102, The Blood Center, 251 Huron Street, Chicago, IL 60611, Phone:
312-926-4325, FAX 312-926-8430, mailto:g-soff@nwu.edu.
TriButyrate® Sodium Phenylbutyrate,
Manufactured by Triple
Crown America, Inc., Phone: 215-453-2500, FAX 215-453-2508, Status:
FDA Approved drug, but not FDA approved for Ewing's Sarcoma. Numerous
preliminary clinical trials in the literature.
*Updated 12/28/2001* REOLYSIN®
is the formulation of the reovirus that is a potential therapeutic for a broad
range of cancers in humans. Reovirus (generic name Respiratory enteric
orphan virus) is an actual benign virus whose use as an antitumor agent is being
advocated by Dr. Patrick Lee, Professor of Microbiology at the University
of Calgary Medical School, Health Sciences Centre, 3330 Hospital Drive,
N.W., Calgary, Alberta, T2N 4N1, Canada, Phone 403-220-7548, FAX 403-270-8520, mailto:plee@ucalgary.ca.
The product is owned by Oncolytics
Biotech Inc., Phone: 403-670-7377, Fax: 403-283-0858 which was acquired in
1999 by Synsorb Biotech Inc.,
201, 1204 Kensington Road, N.W., Calgary, Alberta, T2N 3P5, Canada, Phone
403-283-5900, FAX 403-283-5907. Status: In early phase I clinical trials.
Here is
the news article on the discovery.
Radiation and Photon Treatments Listing:
*New* 9/11/2000 -- Intensity
Modulated Radiation Therapy (IRMT) This is a type of radiation
planning and dosage given that customizes dosage strength and beam paths in 3
dimensions minimizing collateral damage to skin and other organs and maximizing
the efficiency of the dose to the tumor. The write-up on this device from
Oncolink is at this
location. There are several manufacturers of this device. Nomos
appears to have a large number of installations and has an installation
directory off of their main site. Another manufacturer of this device
is Siemens.
A third manufacturer is Varian.
There are several more write-ups on this technique from: Cancer
Treatment Centers of America, University
of Nebraska, Western
Pennsylvania Hospital, University
of Maryland, Science
Daily, and University
of California, Irvine.
Boron Neutron Capture Therapy (BCNT) Pioneered by the
U.S. Government's Brookhaven
National Laboratory Boron Neutron Capture Therapy Research Program
Brookhaven National
Laboratory Medical Department, Jeffrey Coderre Ph.D., mailto:coderre@bnl.gov,
Brookhaven National Laboratory, Medical Department, Upton, NY 11973 USA , Voice:
(631)344-3684, Fax: (631)344-1349. A number of other facilities that have
this technology are listed on the Brookhaven site. BCNT uses drugs containing a
stable isotope of boron, B-10, that are capable of preferentially accumulating
in the tumor, which is then irradiated with low energy (thermal) neutrons.
Another Pioneering laboratory not listed on the Brookhaven site is the Fermilab
Institute for Neutron Therapy, Kirk Road and Pine Street, Batavia, IL
60510-0500, Phone 630-840-3865, FAX 630-840-8766.
Fractionated Stereotactic Body Radiosurgery Staten
Island University Hospital The Radiosurgery Center, Staten Island University
Hospital, 475 Seaview Avenue, Staten Island, New York 10305, Phone:
1-800-285-4584, email
form
Status: A unique method of delivering radiation using multiple beams and angles
that is much healthier for surrounding tissue.
Proton Treatment Loma
Linda University Medical Center Proton Treatment Center, 11234 Anderson
Street, Loma Linda, CA 92354, Phone: 800-776-8667, Fax: 909-824-4829. mailto:referral@dominion.llumc.edu
Status: FDA approved for a number of localized tumor conditions. Protons
are said to be less harmful to other non-tumorous tissue than traditional
radiation. Other proton facilities are available at the Northeast
Proton Therapy Center of the Massachusetts General Hospital, This Harvard
link also has a list of numerous other Proton Treatment facilities. Their Phone
number is: 877-726-5130 or 800-388-4644 or 617-726-5130, E-mail:
infonptc@partners.org
Photon Radiosurgery System Photoelectron
Corp. On September 27, 1999, FDA granted approval to extend the use of
this system to tumors anywhere in the body. The press release is located here.
The Photoelectron phone number is 781-861-2069. This system is basically a
way of using a needle to deliver radiation directly to a tumor with minimal
damage to surrounding tissue.
© Copyright, 1998, 1999, 2000, 2001, Barry Sugarman,
B.S.ENGR., All Rights Reserved.
Phone 310-355-6046, FAX 310-454-9592
e-mail:barry@cureourchildren.org
Return to our Home Page: http://www.cureourchildren.org
This site last updated on February 17, 2002.


